What Is 6.022 X 10 23
penangjazz
Dec 01, 2025 · 10 min read
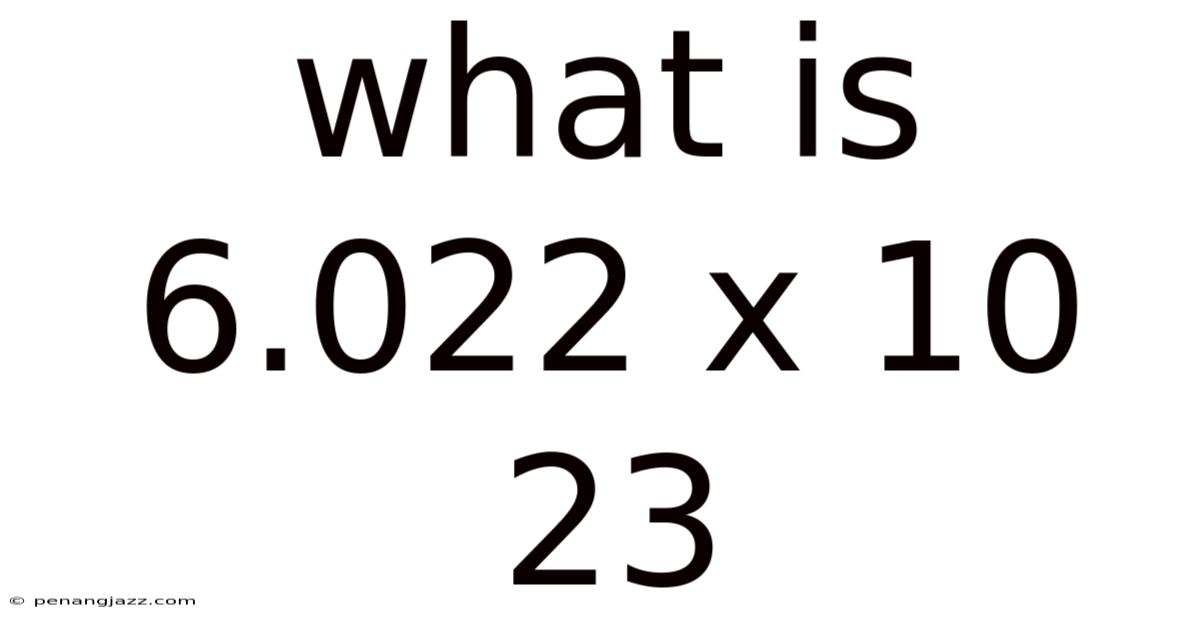
Table of Contents
Unveiling the Mystery of 6.022 x 10^23: More Than Just a Number
The number 6.022 x 10^23, often appearing in scientific contexts, might seem like an arbitrary string of digits. However, this number, known as Avogadro's number, is a fundamental constant in chemistry and physics. It represents a specific quantity of particles, providing a crucial link between the microscopic world of atoms and molecules and the macroscopic world we experience. Understanding Avogadro's number is essential for comprehending concepts like moles, molar mass, and stoichiometry, all of which are cornerstones of chemical calculations.
A Deep Dive into the Concept: Avogadro's Number Explained
Avogadro's number (NA), approximately 6.02214076 × 10^23, defines the number of constituent particles (usually atoms or molecules) that are contained in the amount of substance given by one mole. It's not just a random number; it's meticulously derived and directly related to the definition of the mole, the SI unit for the amount of substance.
The Mole: A Chemist's Counting Unit
To grasp Avogadro's number, we must first understand the concept of the mole. Think of a mole as a chemist's "dozen." Just as a dozen always represents 12 items, a mole always represents 6.022 x 10^23 items. These "items" can be atoms, molecules, ions, electrons, or any other elementary entities.
Why do we need such a large number? Because atoms and molecules are incredibly small. Dealing with individual atoms or molecules in chemical calculations is impractical. The mole provides a convenient way to work with measurable quantities of substances.
Avogadro's Number and the Definition of the Mole
The mole is defined as the amount of a substance that contains as many elementary entities as there are atoms in 12 grams of carbon-12 (¹²C). This is a crucial point. The number of atoms in 12 grams of ¹²C was experimentally determined to be approximately 6.022 x 10^23. Hence, Avogadro's number directly links the mass of a substance to the number of atoms or molecules present.
In simpler terms:
- One mole of any substance contains 6.022 x 10^23 particles of that substance.
- The molar mass of a substance (the mass of one mole) is numerically equal to its atomic or molecular weight expressed in grams per mole (g/mol).
The History Behind the Number: Honoring Avogadro
The concept behind Avogadro's number evolved over time and is not solely attributable to Amedeo Avogadro himself. While the number is named in his honor, he didn't actually determine its value.
Avogadro's Hypothesis (1811): Amedeo Avogadro proposed that equal volumes of all gases, at the same temperature and pressure, contain the same number of molecules. This hypothesis, though initially met with skepticism, laid the groundwork for understanding the relationship between the number of particles and the amount of substance.
The Determination of Avogadro's Number: Numerous scientists contributed to the experimental determination of NA. Methods used include:
- Electrolysis: Measuring the amount of silver deposited during electrolysis and relating it to the charge of a single electron.
- Brownian Motion: Observing the random movement of particles suspended in a fluid, explained by Albert Einstein, which allowed for an estimation of molecular size and number.
- X-ray Crystallography: Using X-rays to determine the spacing between atoms in crystals, allowing for the calculation of the number of atoms in a given volume.
Jean Baptiste Perrin, a French physicist, played a significant role in experimentally confirming Einstein's theory of Brownian motion and using it to determine Avogadro's number. His work was so compelling that it largely convinced the scientific community of the reality of atoms and molecules. Perrin was awarded the Nobel Prize in Physics in 1926 for his work on the discontinuous structure of matter.
Therefore, while Avogadro's name is attached to the number, its determination was a collaborative effort built upon his initial hypothesis.
Why is Avogadro's Number Important? Applications in Chemistry and Beyond
Avogadro's number is not just a theoretical concept; it has profound practical applications in various scientific fields, particularly in chemistry.
1. Stoichiometry: The Language of Chemical Reactions
Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Avogadro's number is indispensable for stoichiometric calculations.
-
Balancing Chemical Equations: Balanced chemical equations represent the molar ratios of reactants and products. For example:
2H₂ + O₂ → 2H₂O
This equation tells us that 2 moles of hydrogen gas (H₂) react with 1 mole of oxygen gas (O₂) to produce 2 moles of water (H₂O). We can then use Avogadro's number to convert these moles to the actual number of molecules involved.
-
Calculating Reactant and Product Quantities: Given the amount of one reactant, we can use the stoichiometric coefficients (the numbers in front of the chemical formulas in the balanced equation) and Avogadro's number to determine the amount of other reactants needed or the amount of products formed.
Example:
Let's say we want to produce 36 grams of water (H₂O). How many grams of hydrogen gas (H₂) are required?
-
Step 1: Convert grams of H₂O to moles: The molar mass of H₂O is approximately 18 g/mol. So, 36 g of H₂O is equal to 36 g / (18 g/mol) = 2 moles of H₂O.
-
Step 2: Use the stoichiometric ratio from the balanced equation: From the equation 2H₂ + O₂ → 2H₂O, we see that 2 moles of H₂O are produced from 2 moles of H₂. Therefore, we need 2 moles of H₂.
-
Step 3: Convert moles of H₂ to grams: The molar mass of H₂ is approximately 2 g/mol. So, 2 moles of H₂ is equal to 2 moles * (2 g/mol) = 4 grams of H₂.
Therefore, we need 4 grams of hydrogen gas to produce 36 grams of water.
2. Determining Molar Mass
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). Avogadro's number connects the atomic mass unit (amu) to grams.
-
Relationship between amu and grams: 1 amu is defined as 1/12th the mass of a carbon-12 atom. Experimentally, it has been determined that 1 amu is approximately equal to 1.66054 x 10⁻²⁴ grams.
-
Calculating Molar Mass: The molar mass of an element is numerically equal to its atomic weight (found on the periodic table) expressed in g/mol. For example, the atomic weight of carbon is approximately 12 amu. Therefore, the molar mass of carbon is approximately 12 g/mol. This means that one mole of carbon atoms (6.022 x 10^23 carbon atoms) has a mass of approximately 12 grams.
For compounds, the molar mass is calculated by summing the atomic weights of all the atoms in the chemical formula. For example, the molar mass of water (H₂O) is (2 * 1 amu for H) + (1 * 16 amu for O) = 18 amu. Therefore, the molar mass of water is approximately 18 g/mol.
3. Gas Laws and Ideal Gas Constant (R)
Avogadro's number plays a role in determining the ideal gas constant (R), which relates the pressure, volume, temperature, and number of moles of an ideal gas.
-
Ideal Gas Law: PV = nRT, where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant
- T is the absolute temperature of the gas
-
Relationship to Avogadro's Number: The ideal gas constant R is related to the Boltzmann constant (k) by the equation R = NA * k, where NA is Avogadro's number. The Boltzmann constant relates the average kinetic energy of particles in a gas to the temperature.
4. Applications in Nanotechnology and Materials Science
In nanotechnology, where materials are manipulated at the atomic and molecular level, Avogadro's number is critical for calculating the number of atoms or molecules required to build specific nanostructures. It allows scientists to precisely control the composition and properties of nanomaterials. Similarly, in materials science, Avogadro's number is used to determine the density and other properties of materials based on their atomic structure.
5. Radiochemistry and Nuclear Physics
Avogadro's number is also used in radiochemistry and nuclear physics. For example, it's used to calculate the specific activity of a radioactive substance, which is the activity per unit mass of the substance. This requires knowing the number of radioactive atoms present in a given mass, which is determined using Avogadro's number.
How is Avogadro's Number Determined? A Glimpse into Experimental Methods
While we've mentioned some historical methods, let's briefly delve into more modern techniques used to determine Avogadro's number.
1. X-ray Diffraction and Crystal Density:
This is one of the most accurate methods. It involves using X-ray diffraction to determine the unit cell volume of a crystal. A unit cell is the smallest repeating unit in a crystal lattice. By knowing the unit cell volume and the density of the crystal, the number of atoms per unit cell can be calculated. From this, Avogadro's number can be derived using the following relationship:
NA = (n * M) / (ρ * V),
where:
- n is the number of atoms per unit cell
- M is the molar mass of the substance
- ρ is the density of the crystal
- V is the volume of the unit cell
2. The Kibble Balance (formerly known as the Watt Balance):
The Kibble balance is an electromechanical measuring instrument that relates mechanical power to electrical power with very high accuracy. It is used to redefine the kilogram based on fundamental constants, including the Planck constant (h). Since the Planck constant is related to other fundamental constants, including the Rydberg constant and the fine-structure constant, and these constants are related to Avogadro’s number, the Kibble balance provides an indirect method for determining Avogadro’s number.
3. Counting Atoms Directly (with limitations):
While not practical for macroscopic quantities, advanced techniques like atom probe tomography allow scientists to image and count individual atoms in small samples. These techniques are valuable for verifying theoretical models and understanding the behavior of materials at the atomic level, even though they can't directly measure Avogadro's number for a mole-sized sample.
Common Misconceptions About Avogadro's Number
-
Avogadro's number is just a large, random number: As explained, it's a meticulously determined constant linked to the definition of the mole and the mass of carbon-12.
-
A mole of everything has the same mass: A mole of different substances will have different masses because their atoms or molecules have different masses. However, a mole of any substance always contains the same number of particles (6.022 x 10^23).
-
Avogadro's number only applies to gases: It applies to any substance, whether solid, liquid, or gas. It's a fundamental constant that relates the amount of substance to the number of constituent particles.
-
Avogadro discovered Avogadro's number: While named in his honor, Amedeo Avogadro proposed the hypothesis that equal volumes of gases contain equal numbers of molecules, but he did not determine the actual value of the number.
The Significance of a Constant Value
The enduring value of Avogadro's number lies in its role as a constant. Constants in science provide stable reference points, allowing for predictable and reliable calculations. Avogadro's number, along with other fundamental constants like the speed of light and the gravitational constant, underpins our understanding of the natural world. Its precise value allows scientists worldwide to communicate and perform experiments with a common frame of reference. It allows for reproducibility, a critical aspect of scientific inquiry.
Conclusion: Appreciating the Immense Yet Intimate Scale
Avogadro's number, 6.022 x 10^23, is far more than just a large number. It's a bridge connecting the microscopic world of atoms and molecules to the macroscopic world we can see and measure. It's a cornerstone of chemistry and physics, enabling us to perform quantitative calculations, understand chemical reactions, and manipulate materials at the atomic level. Understanding this number unlocks a deeper appreciation for the immense yet intimate scale of the universe and the fundamental laws that govern it. By grasping the significance of Avogadro's number, we gain a more profound understanding of the composition of matter and the intricate processes that shape our world.
Latest Posts
Latest Posts
-
What Is A Line Of Poetry
Dec 01, 2025
-
Write The Systematic Name Of Each Organic Molecule
Dec 01, 2025
-
A Battery Uses Energy To Generate Energy
Dec 01, 2025
-
Solving System Of Linear Equation By Substitution Method
Dec 01, 2025
-
What Is The Equation For Population Change
Dec 01, 2025
Related Post
Thank you for visiting our website which covers about What Is 6.022 X 10 23 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.