A Battery Uses _________________ Energy To Generate _______________ Energy.
penangjazz
Dec 01, 2025 · 9 min read
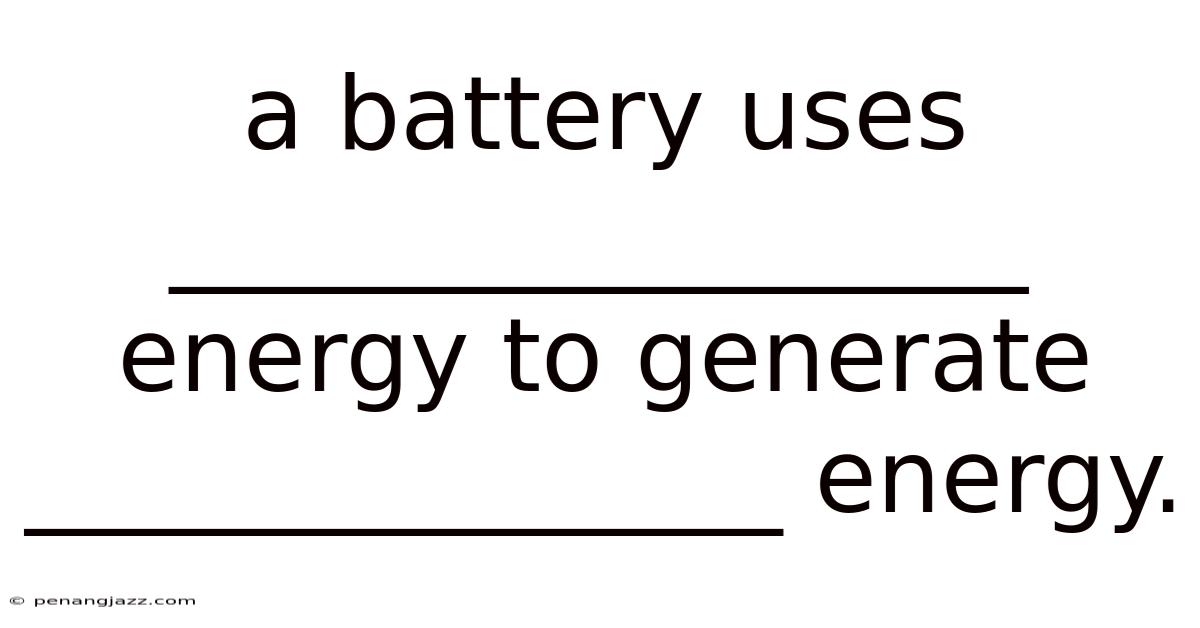
Table of Contents
A battery uses chemical energy to generate electrical energy. This seemingly simple statement is the foundation of a technology that powers our modern world, from smartphones and laptops to electric vehicles and grid-scale energy storage. Understanding the intricacies of this energy conversion, however, requires delving into the fascinating world of electrochemistry, material science, and engineering.
Introduction: The Electrochemical Heart of a Battery
At its core, a battery is an electrochemical device that stores chemical energy and releases it as electrical energy through a controlled chemical reaction. This reaction involves the transfer of electrons between different materials, creating an electric current that can be harnessed to power external devices. The beauty of a battery lies in its ability to perform this conversion reversibly (in rechargeable batteries), allowing us to store and release energy on demand.
The term "battery" itself refers to a collection of one or more electrochemical cells, each containing two electrodes (an anode and a cathode) and an electrolyte. The electrolyte acts as a medium for ion transport between the electrodes, facilitating the flow of charge that drives the electrical circuit. Different types of batteries utilize various chemical reactions and materials, leading to a diverse range of performance characteristics, such as energy density, power output, lifespan, and safety.
A Deep Dive into Battery Components and Functionality
To fully grasp how a battery converts chemical energy into electrical energy, it’s essential to understand the role of each component:
-
Anode (Negative Electrode): This is the electrode where oxidation occurs. Oxidation is the process of losing electrons. During discharge, the anode material releases electrons into the external circuit, becoming positively charged ions that migrate through the electrolyte. Common anode materials include lithium metal, graphite, and various metal alloys.
-
Cathode (Positive Electrode): This is the electrode where reduction occurs. Reduction is the process of gaining electrons. During discharge, the cathode material accepts electrons from the external circuit, becoming negatively charged ions. These ions also migrate through the electrolyte. Common cathode materials include lithium metal oxides, lithium iron phosphate, and sulfur-based compounds.
-
Electrolyte: This is the key to enabling ion transport between the anode and cathode. It's a chemical substance that allows ions to move freely while preventing the flow of electrons. Electrolytes can be liquid (e.g., sulfuric acid in lead-acid batteries), solid (e.g., polymers or ceramics in solid-state batteries), or gel-like. The choice of electrolyte significantly impacts the battery's performance, safety, and operating temperature.
-
Separator: This is a physical barrier that prevents direct contact between the anode and cathode, preventing a short circuit. It's a porous membrane that allows ions to pass through while blocking the passage of electrons.
The Electrochemical Process:
The energy conversion process in a battery can be broken down into the following steps:
-
Chemical Reaction: At the anode, the active material undergoes oxidation, releasing electrons and forming positively charged ions. For example, in a lithium-ion battery, lithium atoms (Li) lose an electron (e-) to become lithium ions (Li+):
Li -> Li+ + e- -
Electron Flow: The released electrons travel through the external circuit, providing electrical energy to power a device.
-
Ion Transport: The lithium ions (Li+) migrate through the electrolyte towards the cathode.
-
Chemical Reaction (Cathode): At the cathode, the lithium ions (Li+) combine with the electrons (e-) arriving from the external circuit and react with the cathode material. For example, lithium ions react with cobalt oxide (CoO2) to form lithium cobalt oxide (LiCoO2):
Li+ + e- + CoO2 -> LiCoO2 -
Circuit Completion: The flow of electrons through the external circuit and the movement of ions through the electrolyte complete the electrical circuit, allowing the battery to discharge and provide power.
In a rechargeable battery, this process is reversed during charging. Electrical energy is supplied to the battery, forcing the electrons to flow in the opposite direction and reversing the chemical reactions at the anode and cathode, restoring the battery to its charged state.
Types of Batteries and Their Chemical Reactions
The specific chemical reactions and materials used vary depending on the type of battery. Here are a few examples:
-
Lead-Acid Batteries: These are the oldest type of rechargeable battery and are commonly used in automobiles. The anode is made of lead (Pb), and the cathode is made of lead dioxide (PbO2). The electrolyte is sulfuric acid (H2SO4). During discharge, lead reacts with sulfuric acid to form lead sulfate (PbSO4) at both electrodes.
- Anode Reaction:
Pb(s) + HSO4-(aq) -> PbSO4(s) + H+(aq) + 2e- - Cathode Reaction:
PbO2(s) + HSO4-(aq) + 3H+(aq) + 2e- -> PbSO4(s) + 2H2O(l)
- Anode Reaction:
-
Nickel-Cadmium (NiCd) Batteries: These batteries were once widely used in portable electronics but have been largely replaced by lithium-ion batteries. The anode is made of cadmium (Cd), and the cathode is made of nickel hydroxide (Ni(OH)2). The electrolyte is potassium hydroxide (KOH).
- Anode Reaction:
Cd(s) + 2OH-(aq) -> Cd(OH)2(s) + 2e- - Cathode Reaction:
2Ni(OH)2(s) + 2e- -> 2Ni(OH)(s) + 2OH-(aq)
- Anode Reaction:
-
Nickel-Metal Hydride (NiMH) Batteries: These batteries offer higher energy density than NiCd batteries and are still used in some hybrid vehicles and portable electronics. The anode is a hydrogen-absorbing alloy, and the cathode is nickel hydroxide (Ni(OH)2). The electrolyte is potassium hydroxide (KOH).
- Anode Reaction:
MH(s) + OH-(aq) -> M(s) + H2O(l) + e-(where MH represents the metal hydride alloy) - Cathode Reaction:
Ni(OH)2(s) + H2O(l) + e- -> Ni(OH)(s) + OH-(aq)
- Anode Reaction:
-
Lithium-Ion (Li-ion) Batteries: These are the most prevalent type of rechargeable battery today, powering everything from smartphones to electric vehicles. The anode is typically made of graphite (carbon), and the cathode is made of a lithium metal oxide (e.g., LiCoO2, LiMn2O4, or LiFePO4). The electrolyte is a lithium salt dissolved in an organic solvent.
- Anode Reaction:
Li(s) -> Li+(sol) + e- - Cathode Reaction:
Li+(sol) + e- + CoO2(s) -> LiCoO2(s)(example using Lithium Cobalt Oxide)
- Anode Reaction:
Factors Affecting Battery Performance
Several factors influence the performance of a battery, including:
-
Electrode Materials: The choice of anode and cathode materials significantly impacts the battery's voltage, capacity, energy density, and lifespan. Materials with high electrochemical potential, high surface area, and good electronic conductivity are preferred.
-
Electrolyte: The electrolyte's ionic conductivity, stability, and compatibility with the electrode materials are crucial for battery performance. Electrolytes with high ionic conductivity allow for faster ion transport, resulting in higher power output.
-
Operating Temperature: Temperature affects the rate of chemical reactions and the conductivity of the electrolyte. Extreme temperatures can degrade battery performance and lifespan.
-
Discharge Rate: The rate at which a battery is discharged affects its capacity and voltage. Higher discharge rates typically result in lower capacity and voltage.
-
Cycle Life: The cycle life refers to the number of charge-discharge cycles a battery can endure before its performance degrades significantly.
The Future of Battery Technology
Battery technology is constantly evolving, with ongoing research and development efforts focused on improving energy density, power output, lifespan, safety, and cost. Some promising areas of research include:
-
Solid-State Batteries: These batteries use solid electrolytes instead of liquid electrolytes, offering improved safety, higher energy density, and wider operating temperature range.
-
Lithium-Sulfur (Li-S) Batteries: These batteries use sulfur as the cathode material, which is abundant and inexpensive. Li-S batteries have the potential to offer significantly higher energy density than Li-ion batteries.
-
Metal-Air Batteries: These batteries use a metal anode (e.g., lithium, zinc, or aluminum) and oxygen from the air as the cathode. Metal-air batteries have the potential to offer extremely high energy density.
-
Flow Batteries: These batteries store energy in liquid electrolytes that are pumped through electrochemical cells. Flow batteries are scalable and can be used for grid-scale energy storage.
-
Sodium-Ion Batteries: Utilizing sodium, an abundant and cheaper element compared to lithium, these batteries present a cost-effective alternative for large-scale energy storage applications.
These advancements in battery technology are crucial for enabling the widespread adoption of electric vehicles, renewable energy sources, and other sustainable technologies.
Environmental Considerations
The production, use, and disposal of batteries have environmental implications. The mining of raw materials, such as lithium and cobalt, can have significant environmental impacts. Battery manufacturing processes can also generate pollution.
Proper battery disposal and recycling are essential to minimize environmental impact. Recycling batteries recovers valuable materials and prevents harmful substances from entering the environment. Many countries and regions have established battery recycling programs to promote responsible battery management.
Applications of Battery Technology
Batteries have become indispensable components in a wide array of applications, driving innovation and shaping numerous industries:
-
Consumer Electronics: Powering smartphones, laptops, tablets, and other portable devices, providing convenience and mobility.
-
Electric Vehicles (EVs): Enabling emission-free transportation, reducing reliance on fossil fuels, and promoting sustainable mobility.
-
Energy Storage Systems (ESS): Storing energy from renewable sources like solar and wind, enhancing grid stability, and enabling off-grid power solutions.
-
Medical Devices: Powering pacemakers, hearing aids, and other life-saving medical devices, improving healthcare outcomes.
-
Aerospace: Powering satellites, drones, and other aerospace applications, facilitating scientific research and exploration.
-
Industrial Equipment: Powering forklifts, power tools, and other industrial equipment, enhancing productivity and efficiency.
-
Backup Power: Providing backup power for critical systems during power outages, ensuring business continuity and safety.
-
Remote and Portable Power: Enabling access to electricity in remote areas and powering portable generators, providing essential services and enhancing quality of life.
Conclusion: The Power Within
Batteries are ingenious devices that harness the power of chemical reactions to generate electrical energy. Understanding the fundamental principles behind their operation, the different types of batteries available, and the factors that influence their performance is crucial for advancing battery technology and addressing the growing demand for energy storage solutions. As we move towards a more sustainable future, batteries will play an increasingly vital role in powering our world. They are more than just power sources; they are enablers of innovation, sustainability, and progress. From the smallest gadgets to the largest energy grids, the impact of batteries is undeniable, and their future potential is limitless. They stand as a testament to human ingenuity, constantly evolving to meet the ever-growing demands of a world increasingly reliant on portable and sustainable energy.
Latest Posts
Latest Posts
-
Fluid And Electrolyte Imbalance Nursing Care Plan
Dec 01, 2025
-
How To Determine The Initial Rate Of Reaction
Dec 01, 2025
-
Define Heat Of Reaction In Chemistry
Dec 01, 2025
-
How Many Electrons Does Fadh2 Carry
Dec 01, 2025
-
Race As A Social Construct Examples
Dec 01, 2025
Related Post
Thank you for visiting our website which covers about A Battery Uses _________________ Energy To Generate _______________ Energy. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.