______________ Have Properties Of Both Metals And Non Metals.
penangjazz
Dec 03, 2025 · 10 min read
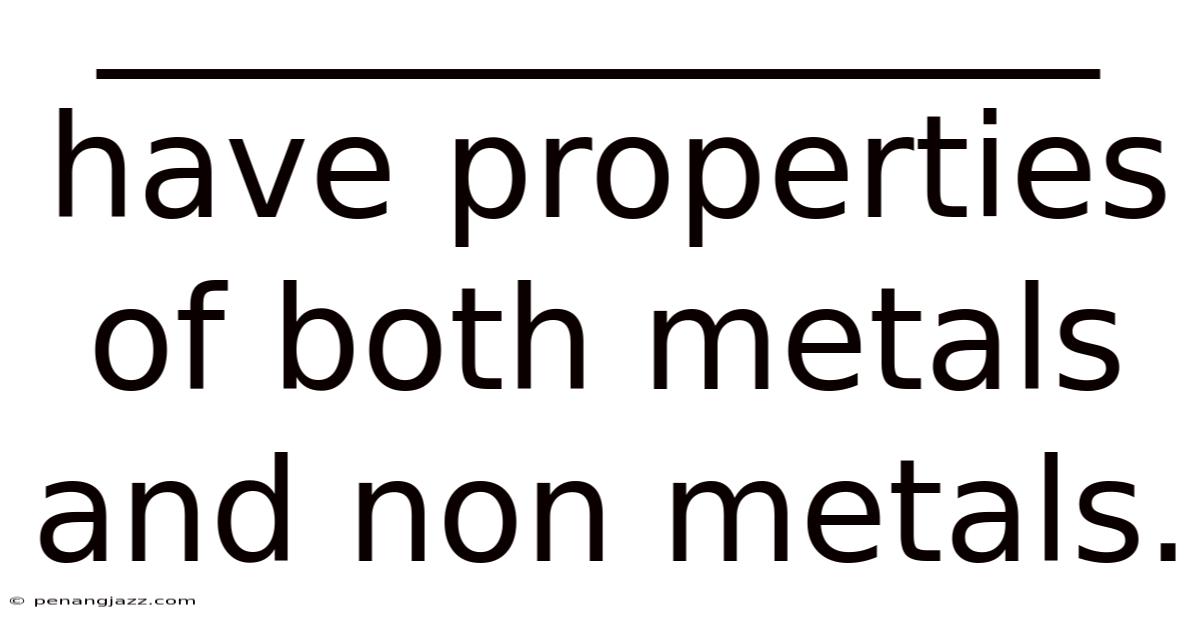
Table of Contents
Metalloids: Bridging the Gap Between Metals and Non-metals
Metalloids, also known as semi-metals, are a fascinating group of elements that possess properties intermediate between those of metals and non-metals. This unique position in the periodic table grants them a wide range of applications in various fields, particularly in the electronics industry. Understanding their properties, classification, and applications is crucial for anyone studying chemistry, materials science, or related fields.
Defining Metalloids: The Elements in Between
Metalloids are elements that exhibit characteristics of both metals and non-metals. There isn't a rigid definition, but generally, metalloids are recognized by their intermediate electrical conductivity, which can be altered by temperature or voltage. This makes them semiconductors, a critical property for electronic devices.
The commonly recognized metalloids include:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
- Polonium (Po) - Sometimes classified as a metalloid
- Astatine (At) - Sometimes classified as a metalloid
The classification of polonium and astatine as metalloids is debated due to their radioactive nature and more metallic properties.
Properties of Metalloids: A Balanced Act
Metalloids showcase a blend of metallic and non-metallic properties, making them unique and versatile. These properties are influenced by their electron configurations and atomic structures.
Physical Properties
- Appearance: Metalloids can exhibit a metallic luster, like silicon, or appear dull, similar to non-metals. Their appearance varies depending on the element and its allotropic form.
- Structure: They generally have a solid-state at room temperature and can form complex structures. For example, boron can form various allotropes, including amorphous and crystalline forms.
- Density and Melting Point: These properties vary among metalloids. Some have high densities and melting points, while others have lower values. For instance, antimony has a relatively high melting point compared to tellurium.
- Hardness and Brittleness: Metalloids are typically harder than metals but more brittle. They lack the malleability and ductility of metals, meaning they cannot be easily hammered into sheets or drawn into wires.
Chemical Properties
- Electronegativity: Metalloids have electronegativity values between those of metals and non-metals. This intermediate electronegativity allows them to form covalent bonds with non-metals and metallic bonds with metals.
- Ionization Energy and Electron Affinity: Their ionization energies and electron affinities are also intermediate. This means they can lose or gain electrons depending on the elements they interact with.
- Oxidation States: Metalloids exhibit a range of oxidation states in their compounds. For example, arsenic can have oxidation states of -3, +3, and +5, depending on the chemical environment.
- Acid-Base Behavior: Metalloids can form oxides that are amphoteric, meaning they can react with both acids and bases. This behavior is another manifestation of their intermediate nature.
Semiconductivity
The most defining property of metalloids is their semiconductivity. Unlike metals, which are excellent conductors, and non-metals, which are insulators, metalloids have an electrical conductivity that falls in between.
- Energy Bands: In solids, electrons occupy energy bands. Metals have overlapping bands, allowing electrons to move freely, thus conducting electricity. Non-metals have a large energy gap between the valence band (where electrons reside) and the conduction band (where electrons can move to conduct electricity), preventing electron flow. Metalloids have a small energy gap, allowing some electrons to jump to the conduction band under certain conditions.
- Temperature Dependence: The conductivity of metalloids increases with temperature. As temperature rises, more electrons gain enough energy to jump the energy gap, increasing conductivity. This is the opposite of metals, where conductivity decreases with temperature due to increased electron scattering.
- Doping: The semiconductivity of metalloids can be precisely controlled by introducing impurities, a process called doping. Adding elements with more valence electrons (n-type doping) or fewer valence electrons (p-type doping) creates an excess of electrons or holes (electron vacancies), respectively, enhancing conductivity.
Individual Metalloids: Properties and Applications
Each metalloid has unique properties and applications, making them valuable in various industries.
Boron (B)
- Properties: Boron is a hard, brittle, black or brown metalloid. It has a high melting point and exists in several allotropic forms. Boron is electron-deficient and tends to form covalent bonds.
- Applications:
- Borosilicate Glass: Boron is used in borosilicate glass, known for its high resistance to thermal shock.
- Boron Fibers: These are used in high-strength, lightweight composites for aerospace applications.
- Boron Carbide: This is an extremely hard material used in abrasives, wear-resistant coatings, and control rods in nuclear reactors.
- Fertilizers: Boron compounds are essential micronutrients for plant growth.
Silicon (Si)
- Properties: Silicon is a gray, lustrous metalloid. It is the most well-known semiconductor and has a tetrahedral crystal structure. Silicon is relatively inert but reacts with halogens and strong alkalis.
- Applications:
- Semiconductors: Silicon is the foundation of the modern electronics industry. It is used to manufacture transistors, integrated circuits, and microchips.
- Solar Cells: Silicon is a key component of photovoltaic cells, which convert sunlight into electricity.
- Silicone Polymers: These are used in lubricants, sealants, adhesives, and medical implants.
- Construction Materials: Silicon compounds are used in concrete and other building materials.
Germanium (Ge)
- Properties: Germanium is a hard, grayish-white metalloid. It has a diamond-like crystal structure and is a semiconductor. Germanium is less abundant than silicon but has higher electron mobility.
- Applications:
- Semiconductors: Germanium was used in early transistors but has largely been replaced by silicon. However, it is still used in some specialized applications.
- Optical Fibers: Germanium dioxide is used in the production of optical fibers for telecommunications.
- Infrared Optics: Germanium is transparent to infrared radiation and is used in infrared detectors and lenses.
- Alloying Agent: Germanium is added to alloys to improve their strength and corrosion resistance.
Arsenic (As)
- Properties: Arsenic is a brittle, steel-gray metalloid. It exists in several allotropic forms, including yellow, black, and gray arsenic. Arsenic is toxic and forms compounds with various oxidation states.
- Applications:
- Alloying Agent: Arsenic is added to lead alloys to increase their hardness and heat resistance.
- Pesticides and Herbicides: Arsenic compounds were historically used as pesticides and herbicides, but their use has declined due to toxicity concerns.
- Wood Preservatives: Copper arsenate is used to preserve wood from decay and insect infestation.
- Semiconductors: Gallium arsenide is a semiconductor used in high-speed electronics and optoelectronic devices.
Antimony (Sb)
- Properties: Antimony is a silvery-white, lustrous metalloid. It is brittle and has a flaky texture. Antimony is a poor conductor of heat and electricity.
- Applications:
- Flame Retardants: Antimony trioxide is used as a flame retardant in plastics, textiles, and paints.
- Alloying Agent: Antimony is added to alloys to improve their hardness, strength, and corrosion resistance.
- Lead-Acid Batteries: Antimony is used in lead-acid batteries to improve their performance and lifespan.
- Pharmaceuticals: Antimony compounds are used in some antiparasitic drugs.
Tellurium (Te)
- Properties: Tellurium is a silvery-white metalloid with a metallic luster. It is brittle and has a hexagonal crystal structure. Tellurium's conductivity increases when exposed to light.
- Applications:
- Alloying Agent: Tellurium is added to steel and copper alloys to improve their machinability.
- Solar Cells: Cadmium telluride is a semiconductor used in thin-film solar cells.
- Rubber Production: Tellurium is used as a vulcanizing agent in rubber production.
- Thermoelectric Devices: Tellurium alloys are used in thermoelectric devices that convert heat into electricity.
Polonium (Po)
- Properties: Polonium is a radioactive metalloid. It is a rare element found in uranium ores. Polonium is highly toxic due to its radioactivity.
- Applications:
- Radioactive Source: Polonium-210 is used as a heat source in radioisotope thermoelectric generators for space applications.
- Antistatic Devices: Polonium was used in antistatic brushes for removing dust from photographic films, but this application has been largely discontinued due to safety concerns.
- Nuclear Weapons: Polonium is used as a neutron trigger in nuclear weapons.
Astatine (At)
- Properties: Astatine is the rarest naturally occurring element and is a radioactive metalloid. Its properties are not well-known due to its scarcity and radioactivity.
- Applications:
- Research: Astatine is primarily used for research purposes, particularly in nuclear medicine.
- Cancer Therapy: Astatine-211 is being investigated for targeted alpha therapy in cancer treatment.
The Role of Metalloids in Semiconductors
The semiconducting properties of metalloids are the foundation of modern electronics. Silicon, in particular, has revolutionized the industry due to its abundance, suitable band gap, and ability to form stable oxides.
-
Doping: The key to controlling the conductivity of semiconductors is doping. Adding impurities with either more or fewer valence electrons than the semiconductor material alters its electrical properties.
- N-type Semiconductors: Doping silicon with elements like phosphorus or arsenic, which have five valence electrons, creates an excess of free electrons. These electrons can move freely through the crystal lattice, increasing conductivity.
- P-type Semiconductors: Doping silicon with elements like boron or gallium, which have three valence electrons, creates "holes" or electron vacancies. Electrons from neighboring atoms can move into these holes, effectively creating a positive charge carrier that contributes to conductivity.
-
P-N Junctions: Combining p-type and n-type semiconductors creates a p-n junction, which is the basic building block of diodes and transistors.
- Diodes: A diode allows current to flow in one direction only. When a positive voltage is applied to the p-side and a negative voltage to the n-side (forward bias), current flows freely. When the voltage is reversed (reverse bias), very little current flows.
- Transistors: Transistors are three-terminal devices that can amplify or switch electronic signals. They are the fundamental components of integrated circuits and microchips.
Advantages and Disadvantages of Using Metalloids
Metalloids offer several advantages in various applications due to their unique properties, but they also have some limitations.
Advantages
- Tunable Conductivity: The ability to control the conductivity of metalloids through doping makes them ideal for semiconductor devices.
- Versatility: Metalloids can form a wide range of compounds with varying properties, making them useful in diverse applications.
- Abundance: Some metalloids, like silicon, are abundant in the Earth's crust, making them cost-effective for large-scale applications.
Disadvantages
- Brittleness: Metalloids are generally brittle and lack the malleability and ductility of metals, limiting their use in structural applications.
- Toxicity: Some metalloids, like arsenic and polonium, are toxic, requiring careful handling and disposal.
- Temperature Sensitivity: The properties of some metalloids can be highly temperature-dependent, which can be a limitation in certain applications.
Future Trends in Metalloid Research
Research on metalloids continues to evolve, with a focus on developing new materials and applications.
- New Semiconductor Materials: Researchers are exploring alternative semiconductor materials beyond silicon, such as germanium, gallium arsenide, and other metalloid-containing compounds. These materials offer the potential for higher electron mobility and improved performance in high-speed electronics.
- Nanomaterials: Metalloid nanoparticles, nanowires, and thin films are being investigated for applications in electronics, photonics, and energy storage.
- Thermoelectric Materials: Researchers are developing new thermoelectric materials based on metalloid alloys for waste heat recovery and energy generation.
- Biomedical Applications: Metalloid-containing compounds are being explored for applications in drug delivery, bioimaging, and cancer therapy.
Conclusion
Metalloids are a fascinating group of elements that bridge the gap between metals and non-metals. Their unique properties, particularly their semiconductivity, make them essential in modern electronics and various other industries. From silicon in microchips to boron in high-strength composites, metalloids play a crucial role in shaping our technological world. As research continues, we can expect to see even more innovative applications of these versatile elements in the future. Their intermediate nature allows for a diverse range of applications, making them indispensable in fields ranging from electronics to materials science. Understanding their properties and applications is critical for advancing technology and addressing global challenges.
Latest Posts
Latest Posts
-
How To Name Cycloalkanes With Substituents
Dec 03, 2025
-
What Is 1 1 3 In A Decimal
Dec 03, 2025
-
Solid Into A Liquid Is Called
Dec 03, 2025
-
What Is R In Population Growth
Dec 03, 2025
-
The Functional Unit Of Kidney Is
Dec 03, 2025
Related Post
Thank you for visiting our website which covers about ______________ Have Properties Of Both Metals And Non Metals. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.