An Enzyme Lowers The __________________________ Of A Chemical Reaction.
penangjazz
Nov 30, 2025 · 12 min read
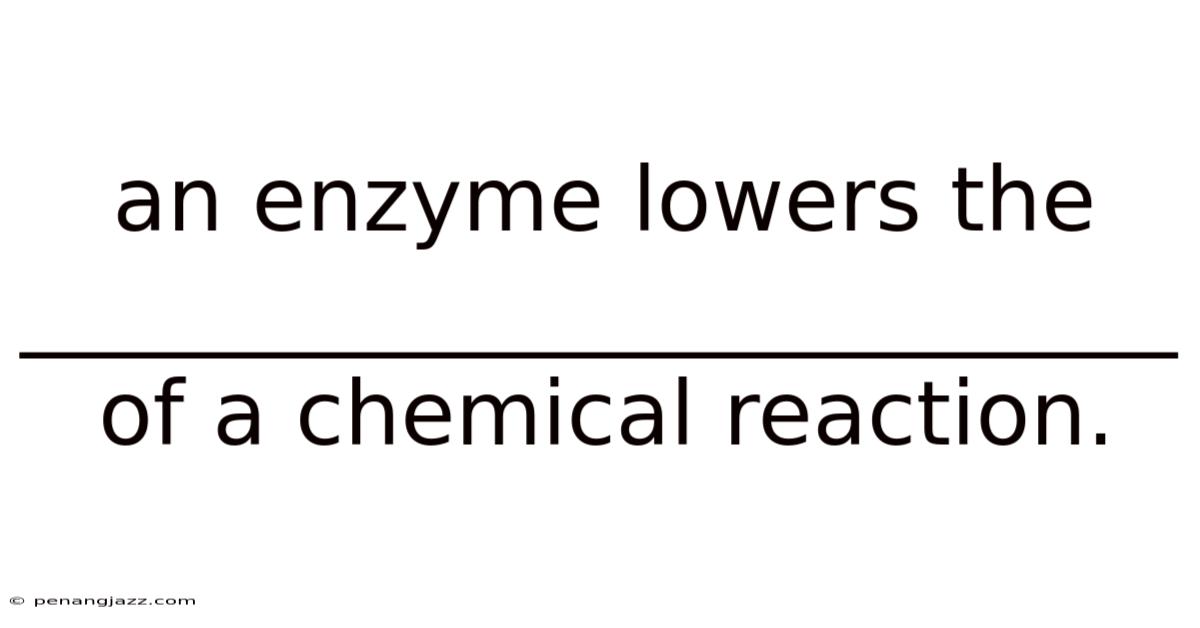
Table of Contents
An enzyme lowers the activation energy of a chemical reaction. This is the fundamental principle that governs how enzymes, the biological catalysts within our bodies and in nature, accelerate life's essential processes. Enzymes are indispensable for virtually every biochemical reaction that occurs, from digesting food to synthesizing DNA. Understanding how they function, particularly their ability to lower activation energy, is crucial for comprehending the intricacies of biology and medicine.
The Essence of Enzymes: Biological Catalysts
Enzymes are specialized proteins that act as catalysts, facilitating and speeding up chemical reactions within living organisms. Unlike non-biological catalysts, enzymes possess a remarkable degree of specificity, meaning each enzyme typically catalyzes only one particular reaction or a set of closely related reactions. This specificity arises from the unique three-dimensional structure of the enzyme, which includes an active site – a region specifically shaped to bind to a particular molecule, known as the substrate.
- Specificity: Enzymes exhibit a high degree of specificity, often catalyzing only a single reaction or a small set of similar reactions.
- Efficiency: Enzymes can accelerate reaction rates by factors of millions or even billions compared to uncatalyzed reactions.
- Regulation: Enzyme activity is tightly regulated within cells, ensuring that reactions occur only when and where they are needed.
- Reusability: Enzymes are not consumed or permanently altered during the reactions they catalyze. They can participate in numerous reaction cycles.
Understanding Activation Energy: The Energy Barrier
Before delving into how enzymes lower activation energy, it's essential to understand what activation energy is in the first place.
In any chemical reaction, molecules need a certain amount of energy to initiate the process. This energy is called activation energy (Ea). It's the energy required to reach the transition state, an intermediate state between reactants and products where chemical bonds are being broken and formed.
Think of it like pushing a rock over a hill. The rock represents the reactants, the hill represents the activation energy barrier, and the other side of the hill represents the products. You need to exert a certain amount of energy (pushing the rock) to get it over the crest of the hill (the transition state) so it can roll down the other side (forming the products). The higher the hill (the higher the activation energy), the harder it is to push the rock over.
Several factors contribute to activation energy:
- Bond breaking: Energy is needed to break the existing chemical bonds within the reactant molecules.
- Molecular orientation: Reactant molecules must collide with the correct orientation for the reaction to occur. Achieving this correct orientation requires energy.
- Solvation effects: In solution, solvent molecules can stabilize reactant molecules, requiring energy to disrupt these interactions.
Reactions with high activation energies tend to be slow because only a small fraction of molecules possesses enough energy to overcome the barrier at any given time. Conversely, reactions with low activation energies proceed more rapidly.
How Enzymes Lower Activation Energy: The Catalytic Mechanism
Enzymes accelerate reactions by lowering the activation energy needed to reach the transition state. They achieve this through a variety of mechanisms:
-
Stabilizing the Transition State: The most crucial way enzymes lower activation energy is by stabilizing the transition state. The active site of the enzyme is precisely shaped to complement the transition state structure of the reaction. When the substrate binds to the active site, the enzyme undergoes conformational changes that further stabilize the transition state. This stabilization lowers the energy difference between the reactants and the transition state, effectively reducing the activation energy.
Imagine the enzyme as a mold specifically designed to fit the transition state molecule. By perfectly cradling the transition state, the enzyme lowers its energy and makes it easier to form.
-
Providing an Alternative Reaction Pathway: Enzymes can provide an alternative reaction pathway with a lower activation energy. Instead of the reaction proceeding through the inherently high-energy pathway, the enzyme facilitates a different sequence of steps with lower energy requirements.
Think of it as the enzyme digging a tunnel through the hill instead of pushing the rock over the top. The tunnel represents the alternative reaction pathway with a lower activation energy barrier.
-
Bringing Reactants Together (Proximity and Orientation Effects): Enzymes increase the local concentration of reactants at the active site, bringing them into close proximity. They also orient the reactants in the optimal configuration for the reaction to occur. This reduces the entropic penalty associated with bringing the reactants together and aligning them properly, effectively lowering the activation energy.
Enzymes act as molecular matchmakers, bringing the reactants together in the right place at the right time and in the correct orientation. This significantly increases the likelihood of a successful reaction.
-
Straining Bonds: Some enzymes physically strain or distort the bonds in the substrate molecule, making them easier to break. This straining reduces the energy required to reach the transition state, lowering the activation energy.
This is like pre-weakening a piece of wood before trying to break it. The enzyme weakens the bonds in the substrate, making it easier for the reaction to occur.
-
Providing a Microenvironment: The active site of an enzyme can provide a specific microenvironment that favors the reaction. This microenvironment may involve:
- pH optimization: Enzymes often have optimal activity at specific pH values. The active site may contain amino acid residues that act as acids or bases, facilitating proton transfer reactions.
- Exclusion of water: Water molecules can sometimes interfere with certain reactions. Some enzymes create a hydrophobic microenvironment at the active site, excluding water and promoting the reaction.
- Metal ion catalysis: Some enzymes require metal ions as cofactors to catalyze the reaction. These metal ions can act as Lewis acids, stabilizing negative charges in the transition state or facilitating redox reactions.
A Visual Representation: Energy Diagrams
Energy diagrams, also known as reaction coordinate diagrams, are powerful tools for visualizing the effect of enzymes on activation energy. These diagrams plot the energy of the system as the reaction progresses from reactants to products.
-
Uncatalyzed Reaction: In an uncatalyzed reaction, the energy diagram shows a single peak representing the activation energy barrier. The height of this peak corresponds to the activation energy (Ea) required for the reaction to occur.
-
Enzyme-Catalyzed Reaction: In an enzyme-catalyzed reaction, the energy diagram shows a lower peak compared to the uncatalyzed reaction. This indicates that the activation energy (Ea) has been reduced by the enzyme. The difference in height between the two peaks represents the decrease in activation energy achieved by the enzyme. The catalyzed reaction may also show multiple smaller peaks, corresponding to the different steps in the enzymatic mechanism.
By comparing the energy diagrams of catalyzed and uncatalyzed reactions, it becomes clear that enzymes significantly lower the activation energy, making the reaction proceed much faster.
Examples of Enzyme Action in Biological Systems
The principle of enzymes lowering activation energy is fundamental to countless biological processes. Here are just a few examples:
- Digestion: Digestive enzymes like amylase (breaks down carbohydrates), protease (breaks down proteins), and lipase (breaks down fats) lower the activation energy required to hydrolyze the bonds in these macromolecules. This allows us to efficiently break down food into smaller molecules that can be absorbed into the bloodstream.
- DNA Replication: DNA polymerase, the enzyme responsible for replicating DNA, lowers the activation energy required to add nucleotides to the growing DNA strand. This ensures that DNA replication occurs with high speed and accuracy.
- Cellular Respiration: Enzymes involved in cellular respiration, such as those in glycolysis, the citric acid cycle, and the electron transport chain, lower the activation energies of the various oxidation-reduction reactions that generate ATP, the cell's primary energy currency.
- Photosynthesis: Enzymes in the Calvin cycle of photosynthesis lower the activation energy required to fix carbon dioxide into sugars. This allows plants to convert light energy into chemical energy.
- Muscle Contraction: Myosin ATPase, the enzyme responsible for powering muscle contraction, lowers the activation energy required to hydrolyze ATP, providing the energy needed for the myosin head to bind to actin and pull the muscle fibers.
Factors Affecting Enzyme Activity
While enzymes are highly efficient catalysts, their activity can be affected by several factors:
-
Temperature: Enzymes have an optimal temperature range for activity. At low temperatures, enzyme activity is slow. As temperature increases, activity increases until the optimal temperature is reached. Above the optimal temperature, the enzyme denatures (loses its shape) and activity decreases rapidly.
-
pH: Enzymes also have an optimal pH range for activity. Changes in pH can affect the ionization state of amino acid residues in the active site, altering the enzyme's ability to bind to the substrate and catalyze the reaction.
-
Substrate Concentration: As substrate concentration increases, enzyme activity increases until it reaches a maximum rate (Vmax). At Vmax, all enzyme active sites are saturated with substrate, and adding more substrate will not increase the reaction rate.
-
Enzyme Concentration: As enzyme concentration increases, the reaction rate increases proportionally, provided that there is sufficient substrate available.
-
Inhibitors: Inhibitors are molecules that decrease enzyme activity. They can bind to the enzyme's active site (competitive inhibitors) or to a different site on the enzyme (non-competitive inhibitors), altering its shape and reducing its catalytic efficiency.
-
Activators: Activators are molecules that increase enzyme activity. They can bind to the enzyme and increase its affinity for the substrate or increase the catalytic efficiency of the enzyme.
Enzymes in Medicine and Industry
The understanding of how enzymes lower activation energy has led to numerous applications in medicine and industry:
-
Drug Development: Many drugs are designed to inhibit specific enzymes involved in disease processes. For example, statins, which are used to lower cholesterol, inhibit the enzyme HMG-CoA reductase, a key enzyme in cholesterol synthesis.
-
Diagnostics: Enzymes are used in diagnostic tests to detect and measure various substances in the body. For example, measuring the levels of certain enzymes in the blood can help diagnose heart attacks, liver disease, and other conditions.
-
Industrial Biotechnology: Enzymes are widely used in industrial processes such as:
- Food processing: Enzymes are used to produce high-fructose corn syrup, clarify fruit juices, and improve the texture of baked goods.
- Detergent industry: Enzymes are added to detergents to break down stains and improve cleaning efficiency.
- Textile industry: Enzymes are used to bleach and soften fabrics.
- Biofuel production: Enzymes are used to break down cellulose into sugars, which can then be fermented into ethanol.
The Future of Enzyme Research
Enzyme research continues to be a vibrant and exciting field with many promising avenues for future exploration:
-
Enzyme Engineering: Scientists are using techniques like directed evolution and rational design to create enzymes with improved stability, activity, and specificity for specific applications.
-
Metabolic Engineering: By manipulating the expression of specific enzymes in metabolic pathways, scientists can engineer organisms to produce valuable products such as pharmaceuticals, biofuels, and bioplastics.
-
Synthetic Biology: Enzymes are being used as building blocks in synthetic biology to create artificial metabolic pathways and novel biological systems.
-
Nanotechnology: Enzymes are being integrated into nanoscale devices for applications such as biosensing, drug delivery, and bioremediation.
Conclusion: The Power of Enzyme Catalysis
Enzymes are essential for life, and their ability to lower the activation energy of chemical reactions is the key to their remarkable catalytic power. By stabilizing the transition state, providing alternative reaction pathways, bringing reactants together, straining bonds, and providing a specific microenvironment, enzymes accelerate reactions by factors of millions or even billions. Understanding these mechanisms has led to numerous applications in medicine, industry, and biotechnology, and continues to drive exciting new research in the field. Enzymes are the workhorses of the biological world, and their continued study will undoubtedly lead to further breakthroughs in our understanding of life and the development of new technologies. The principle that an enzyme lowers the activation energy of a chemical reaction remains a cornerstone of modern biochemistry and a testament to the elegance and efficiency of nature's design.
Frequently Asked Questions (FAQ)
-
What happens if an enzyme doesn't lower the activation energy? If an enzyme couldn't lower the activation energy, the reaction it catalyzes would proceed at a much slower rate, likely too slow to be biologically relevant. The enzyme's role is to accelerate reactions that would otherwise be too slow to sustain life processes. Without the enzyme's catalytic effect, the reaction would either not occur at a useful rate or would require extreme conditions (like high temperatures or pressures) that are incompatible with living systems.
-
Are there enzymes that increase activation energy? No, enzymes do not increase activation energy. Enzymes are catalysts, and by definition, catalysts speed up reactions by lowering the activation energy. A molecule that increased the activation energy would inhibit the reaction, not catalyze it.
-
How do cofactors and coenzymes affect enzyme activity? Cofactors (inorganic ions like magnesium or zinc) and coenzymes (organic molecules like vitamins) can assist enzymes in lowering activation energy. They often participate directly in the reaction mechanism or help to stabilize the enzyme's structure, making it more effective at binding substrates and facilitating the reaction.
-
Can temperature denature enzymes, and how does this affect activation energy? Yes, high temperatures can denature enzymes, causing them to lose their specific three-dimensional structure. Denaturation disrupts the active site, making the enzyme unable to bind substrates effectively or stabilize the transition state. This increases the activation energy required for the reaction because the enzyme is no longer able to provide its catalytic effect.
-
Is the active site of an enzyme rigid or flexible? The active site of an enzyme is generally flexible. The induced fit model suggests that the active site changes shape upon substrate binding to achieve optimal interaction with the substrate and stabilization of the transition state. This flexibility is crucial for the enzyme's ability to lower activation energy effectively.
-
Do all enzymes have the same efficiency in lowering activation energy? No, enzymes vary in their efficiency at lowering activation energy. Some enzymes can lower the activation energy more significantly than others, leading to faster reaction rates. The efficiency depends on the enzyme's specific structure, active site environment, and the mechanisms it employs to catalyze the reaction.
Latest Posts
Latest Posts
-
How To Transcribe Dna To Rna
Dec 01, 2025
-
Partial Pressure Of Co2 In Atmosphere
Dec 01, 2025
-
Difference Between Prokaryotic And Eukaryotic Gene Expression
Dec 01, 2025
-
How Is Kinetic Energy Related To Temperature
Dec 01, 2025
-
How To Determine If The Function Is One To One
Dec 01, 2025
Related Post
Thank you for visiting our website which covers about An Enzyme Lowers The __________________________ Of A Chemical Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.