1s 2 2s 2 2p 6
penangjazz
Nov 20, 2025 · 11 min read
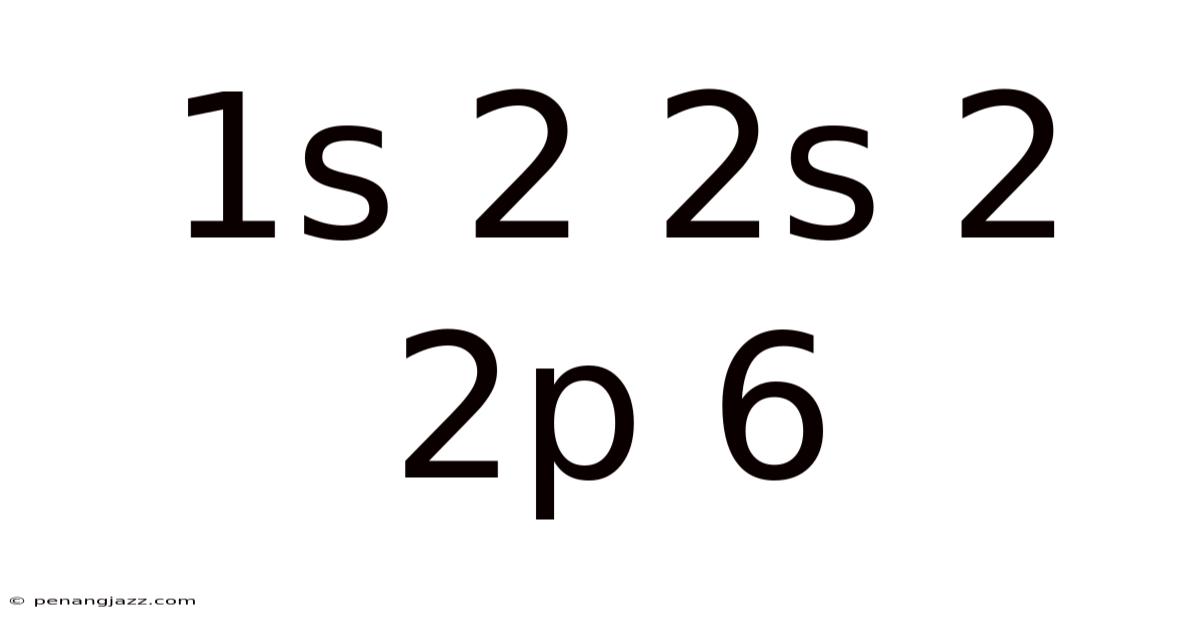
Table of Contents
Diving into the heart of chemistry and physics, the sequence 1s² 2s² 2p⁶ unlocks a fundamental understanding of atomic structure. This seemingly simple string of characters represents the electron configuration of a specific element, dictating its chemical behavior and properties. To truly grasp its significance, we need to break down each component and explore the principles governing the arrangement of electrons within an atom.
Understanding Electron Configuration: The Foundation
Electron configuration provides a roadmap of how electrons, the negatively charged particles orbiting the nucleus of an atom, are arranged within different energy levels and sublevels. These arrangements are not arbitrary; they follow specific rules dictated by quantum mechanics, a branch of physics that describes the behavior of matter at the atomic and subatomic levels. The 1s² 2s² 2p⁶ configuration specifically describes the arrangement of electrons in a filled neon atom, showcasing a stable and unreactive state.
Before we dissect the configuration itself, let's review some crucial concepts:
- Electrons: Negatively charged particles that orbit the nucleus of an atom. They are the key players in chemical bonding and determine an element's reactivity.
- Energy Levels (Shells): Electrons occupy specific energy levels, often visualized as shells surrounding the nucleus. These levels are numbered 1, 2, 3, and so on, with level 1 being closest to the nucleus and having the lowest energy.
- Sublevels (Subshells): Within each energy level, electrons reside in sublevels, denoted by the letters s, p, d, and f. Each sublevel has a specific shape and energy.
- Orbitals: Each sublevel consists of one or more orbitals. An orbital is a region of space around the nucleus where there is a high probability of finding an electron. Each orbital can hold a maximum of two electrons.
- Aufbau Principle: Electrons fill orbitals in order of increasing energy. This principle provides a guideline for predicting electron configurations.
- Hund's Rule: Within a given sublevel, electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion and results in a more stable configuration.
- Pauli Exclusion Principle: No two electrons in the same atom can have the same set of four quantum numbers. This implies that each orbital can hold a maximum of two electrons, and these electrons must have opposite spins.
Decoding 1s² 2s² 2p⁶: A Step-by-Step Analysis
Now that we have the fundamental principles in place, let's decipher the meaning of the 1s² 2s² 2p⁶ electron configuration:
- 1s²: The "1" indicates the first energy level (the innermost shell). The "s" denotes the s sublevel, which is spherical in shape and can hold a maximum of two electrons. The superscript "²" signifies that this s sublevel is completely filled with two electrons.
- 2s²: The "2" indicates the second energy level. The "s" again denotes the s sublevel. The superscript "²" indicates that this s sublevel in the second energy level is also filled with two electrons.
- 2p⁶: The "2" still indicates the second energy level. The "p" denotes the p sublevel. The p sublevel has a dumbbell shape and consists of three orbitals, each of which can hold two electrons. Therefore, the p sublevel can hold a maximum of six electrons. The superscript "⁶" indicates that all three p orbitals in the second energy level are completely filled with six electrons.
In summary, the 1s² 2s² 2p⁶ configuration tells us that the atom has two electrons in its first energy level (in the s sublevel) and eight electrons in its second energy level (two in the s sublevel and six in the p sublevel). This configuration accounts for a total of 10 electrons.
The Significance of a Full Outer Shell: Neon and Noble Gases
The 1s² 2s² 2p⁶ configuration is the electron configuration of neon (Ne), a noble gas. Noble gases are characterized by their exceptional stability and lack of reactivity. This stability arises from having a full outermost electron shell, also known as the valence shell.
The octet rule, a simplification of more complex quantum mechanical principles, states that atoms tend to gain, lose, or share electrons in order to achieve a full valence shell with eight electrons (except for hydrogen and helium, which strive for two electrons). Neon already possesses a full valence shell with eight electrons (2s² 2p⁶), making it exceptionally stable and resistant to forming chemical bonds with other atoms. This explains why neon exists as a monatomic gas and is used in applications where inertness is crucial, such as in neon lights.
Other noble gases, such as helium (He), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), also have full valence shells, making them similarly unreactive. Their electron configurations follow the same pattern, with filled s and p sublevels in their outermost shells.
Beyond Neon: Electron Configuration and the Periodic Table
The concept of electron configuration is intimately linked to the periodic table. The periodic table is organized in such a way that elements with similar electron configurations and, therefore, similar chemical properties are grouped together.
- Periods (Rows): The period number corresponds to the highest energy level occupied by electrons in the element's electron configuration. For example, neon is in the second period, and its electron configuration shows that the highest energy level occupied is the second energy level (n=2).
- Groups (Columns): Elements within the same group (vertical column) have the same number of valence electrons, leading to similar chemical behavior. For example, all noble gases (Group 18) have a full valence shell, making them unreactive.
Knowing the electron configuration of an element allows us to predict its position in the periodic table and, consequently, its chemical properties. Conversely, knowing an element's position in the periodic table provides valuable clues about its electron configuration.
The filling of electron orbitals follows a specific pattern that dictates the structure of the periodic table:
- s-block: Groups 1 and 2 (alkali metals and alkaline earth metals) are in the s-block. Their valence electrons occupy s orbitals.
- p-block: Groups 13-18 are in the p-block. Their valence electrons occupy p orbitals.
- d-block: Groups 3-12 (transition metals) are in the d-block. Their valence electrons occupy d orbitals.
- f-block: The lanthanides and actinides are in the f-block. Their valence electrons occupy f orbitals.
This relationship between electron configuration and the periodic table is a cornerstone of chemistry, allowing us to understand and predict the behavior of elements and their interactions.
Determining Electron Configurations: Rules and Examples
While the Aufbau principle, Hund's rule, and the Pauli exclusion principle provide a general framework for determining electron configurations, there are some exceptions and nuances to consider.
Here's a systematic approach to writing electron configurations:
- Determine the number of electrons: This is equal to the atomic number of the element.
- Apply the Aufbau principle: Fill the orbitals in order of increasing energy: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. A helpful mnemonic is to use the diagonal rule, where you write the orbitals in order of increasing energy level and then draw diagonal lines to indicate the filling order.
- Apply Hund's rule: Within a given sublevel (e.g., p sublevel), electrons will individually occupy each orbital before pairing up.
- Apply the Pauli exclusion principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Write the electron configuration: Summarize the number of electrons in each sublevel using the notation described earlier (e.g., 1s², 2s², 2p⁶).
Examples:
- Hydrogen (H): Atomic number = 1. Electron configuration: 1s¹
- Oxygen (O): Atomic number = 8. Electron configuration: 1s² 2s² 2p⁴
- Sodium (Na): Atomic number = 11. Electron configuration: 1s² 2s² 2p⁶ 3s¹
- Iron (Fe): Atomic number = 26. Electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
Shorthand Notation:
For elements with many electrons, it's common to use a shorthand notation that utilizes the electron configuration of the preceding noble gas. For example, sodium (Na) can be written as [Ne] 3s¹, where [Ne] represents the electron configuration of neon (1s² 2s² 2p⁶). This simplifies the notation and highlights the valence electrons, which are most important for chemical bonding.
Exceptions to the Aufbau Principle:
While the Aufbau principle is a useful guideline, there are exceptions, particularly among the transition metals. These exceptions arise from the subtle energy differences between orbitals and the tendency of atoms to achieve half-filled or fully-filled d sublevels, which are particularly stable.
For example, chromium (Cr) has an expected electron configuration of [Ar] 4s² 3d⁴, but its actual electron configuration is [Ar] 4s¹ 3d⁵. This is because a half-filled d sublevel (3d⁵) is more stable than a partially filled d sublevel (3d⁴) with a filled s sublevel (4s²). Similarly, copper (Cu) has an expected electron configuration of [Ar] 4s² 3d⁹, but its actual electron configuration is [Ar] 4s¹ 3d¹⁰, achieving a fully filled d sublevel (3d¹⁰).
The Role of Electron Configuration in Chemical Bonding
Electron configuration plays a crucial role in determining how atoms interact with each other to form chemical bonds. The valence electrons, those in the outermost shell, are the ones involved in bonding.
There are two main types of chemical bonds:
- Ionic Bonds: These bonds form through the transfer of electrons between atoms. Typically, a metal atom loses electrons to form a positive ion (cation), and a nonmetal atom gains electrons to form a negative ion (anion). The electrostatic attraction between the oppositely charged ions holds the bond together. The electron configurations of the resulting ions are often isoelectronic (having the same electron configuration) with noble gases, reflecting their enhanced stability.
- Covalent Bonds: These bonds form through the sharing of electrons between atoms. Covalent bonds typically occur between nonmetal atoms. The shared electrons are attracted to the nuclei of both atoms, holding them together. The electron configurations of the bonded atoms are altered to achieve a more stable state, often resembling a noble gas configuration through shared electrons.
Understanding electron configuration allows us to predict the types of bonds that will form between different atoms and the properties of the resulting compounds. For instance, elements with a strong tendency to lose electrons (e.g., alkali metals) will readily form ionic bonds with elements with a strong tendency to gain electrons (e.g., halogens). Elements that are close to achieving a full octet by sharing electrons (e.g., carbon) will tend to form covalent bonds with other nonmetals.
Applications of Electron Configuration
The knowledge of electron configuration has numerous applications in various fields:
- Predicting Chemical Properties: Electron configuration is the key to understanding and predicting the chemical behavior of elements and their compounds.
- Spectroscopy: The electron configuration of an atom determines its absorption and emission spectra. Spectroscopy is a powerful technique used to identify elements and molecules based on their unique spectral fingerprints.
- Materials Science: Electron configuration influences the electronic and magnetic properties of materials. Understanding electron configuration is crucial for designing new materials with desired properties for applications in electronics, energy storage, and other fields.
- Quantum Chemistry: Electron configuration is a fundamental concept in quantum chemistry, which uses quantum mechanics to study the structure and properties of molecules.
Common Misconceptions
- Electrons Orbit the Nucleus in Fixed Paths: A common misconception is that electrons orbit the nucleus in fixed, well-defined paths, similar to planets orbiting the sun. In reality, electrons occupy regions of space called orbitals, which represent the probability of finding an electron in a particular location.
- The Octet Rule is Universal: The octet rule is a useful guideline, but it is not universally applicable. There are many exceptions, particularly for elements beyond the second period.
- Electron Configuration is Static: Electron configuration represents the ground state (lowest energy state) of an atom. Electrons can be excited to higher energy levels by absorbing energy, leading to changes in the electron configuration.
Conclusion
The electron configuration 1s² 2s² 2p⁶ is more than just a string of characters; it's a window into the fundamental structure and behavior of atoms. By understanding the principles governing electron arrangement, we gain a powerful tool for predicting chemical properties, understanding bonding, and designing new materials. From the stability of neon to the reactivity of sodium, electron configuration provides a framework for understanding the vast diversity of the chemical world. Mastering this concept is essential for anyone seeking a deeper understanding of chemistry and the world around us. The principles discussed extend beyond the simple example of neon and provide the foundation for understanding the electronic structure of all elements and their resulting properties.
Latest Posts
Latest Posts
-
What Type Of Compounds Dissolve In Water
Nov 20, 2025
-
How To Calculate The Mean From A Frequency Table
Nov 20, 2025
-
Charging And Discharging Of Capacitor Formula
Nov 20, 2025
-
What Has More Electrolytes Gatorade Or Powerade
Nov 20, 2025
-
Where Does The First Step Of Protein Synthesis Occur
Nov 20, 2025
Related Post
Thank you for visiting our website which covers about 1s 2 2s 2 2p 6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.